A biological drug is defined as a: …substance that is made from a living organism or its products and is used in the prevention, diagnosis, or treatment of cancer and other diseases. Biological drugs include antibodies, interleukins, and vaccines. The market for...
process validation
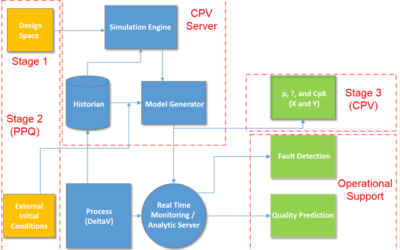
Continued Process Verification in the Process Validation Lifecycle
In 2011, the U.S. Food and Drug Administration (FDA) issued a Guidance for Industry – Process Validation: General Principles and Practices. It highlighted a third validation stage goal of continued process verification (CPV) for: …continual assurance that the process...
Continuous Process Verification per FDA Process Validation Guidance
It was just about a year ago that the U.S. Food and Drug Administration published their Guidance for Industry - Process Validation: General Principles and Practices. I caught up with Emerson's Heather Schwalje, a senior consultant on the Life Sciences industry team....
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.