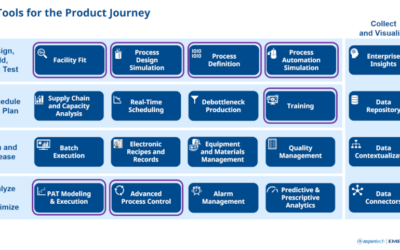
On June 4-7, experts in process analytical technology (PAT) gathered for the 2023 IFPAC conference. While there, they discussed the latest developments in PAT, quality by design, and overall process monitoring and control within the pharmaceutical, biotechnology,...
life sciences
Data-Driven Culture Drives Maintenance and Reliability Results
Life sciences organizations are not short on data. Most plants already have many sensors in place to drive operational data to the control system so operators always know exactly what is happening at any given moment of production. Operations teams know that good data...
Digital Solutions for Process Development, Tech Transfer and Manufacturing
Emerson’s Michalle Adkins and AspenTech’s Chuck Miller team up to present Robust PAT Solutions to Support Process Development, Tech Transfer and Manufacturing at IFPAC 2023.
Traceability Across the Entire Drug Development Pipeline
Recently, as cell and gene therapies have begun to emerge in the life sciences manufacturing landscape, an important concept has begun to draw attention: traceability. However, it is important to remember that traceability was critical long before today’s most...
Better Treatment—For the Earth, and its People
It can be hard to keep up with the many rapid changes occurring in the life sciences industry these days. In an effort to embrace improved speed to market, sustainability, and quality, life sciences manufacturers are employing a wide range of software and technologies...
Four Pillars of Life Sciences Speed to Market
People are more aware than ever of the many amazing ways new treatments can improve their lives. As a result, life sciences companies are under more pressure than ever to safely deliver new, innovative treatments to market to satisfy customer demand. The key to...
Smart Maintenance Practices in Biopharmaceutical Manufacturing
In a new paper published by the BioPhorum organization, Smart Maintenance Architecture: A Data-Driven Approach to Smarter Maintenance, Emerson’s Jonas Berge joined with experts from Applied Materials, Bristol Myers Squibb, GSK, Merck, Roche, and members of the BioPhorum team to collaborate and publish this paper.
Improving Digital Maturity is Key to Life Sciences Success
In just the last 10 years, biopharmaceutical supply chains have changed dramatically. The single-source manufacturing model is becoming much rarer, making way for multi-enterprise manufacturing collaborations and partnerships to better meet the changing needs of the...
Leverage Life Sciences Software to Reduce Time to Market
The days of maintaining paper records across the life sciences development and production pipeline are gone. Today’s regulatory requirements, not to mention the complexity of new treatments, requires organization that can only be provided electronically. However,...
Module Type Package Unlocks the Flexibility Driving Pharma Innovation
There can be little question that Industry 4.0 technologies are reshaping the way life sciences companies operate. In the wake of the rapid development of vaccines during the COVID-19 pandemic, more facilities than ever are being “born digital.” Some of these...
Let your Personnel Lead you to the Adaptive Plant
On Monday at the Emerson Exchange Users’ Conference, Emerson’s Michalle Adkins and AbbVie’s Jeff Ziolkowski moderated a panel with Fujifilm’s Soren Skerris, Takeda’s Dr. Nina Schwalb, Eli Lilly’s James Weber, and Emerson’s Christian Berg. The experts shared the...
Software Simplifies Technology Transfer
The life sciences marketplace is changing faster than nearly any other industry. Gone are the days when companies focus on a single treatment to drive their entire business. Today’s life sciences manufacturers are challenged to bring new treatments to the marketplace...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.