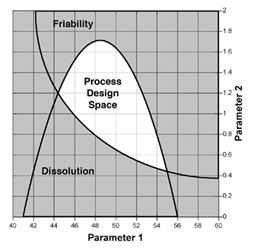
As the landscape of pharmaceutical manufacturing has changed in the last few years, one exciting shift has a distinct possibility of being a game changer in coming decades. Regulatory agencies have increasingly begun to recognize the importance of automation...
FDA
Successfully Implementing Process Analytical Technology
It's been well more than a decade since the U.S. Food & Drug Administration (FDA) announced a Process Analytical Technology (PAT) approach for pharmaceutical manufacturers in their Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development,...
US FDA Quality Metrics Guidelines Update
Author: Eric Kuebler Since the Continued Process Verification (CPV) guidance in 2011, the U.S. Food and Drug Administration (FDA) has been talking quality and verifying process state of control. Now the FDA is getting around to defining what that looks like and what...
Implementing Quality by Design
Many manufacturers in the Life Sciences industry are challenged to respond to a new paradigm for process development and manufacturing. The FDA's cGMP for the 21st Century initiative is driving the industry to change its development and manufacturing to be based on...
CPhI Pharma Evolution Guest Post-Electronic Batch Records: Are Your Systems Ready for Inspection?
I wanted to share my guest post published at the CPhI Pharma Evolution website. There are some great comments, so join in if the post sparks some ideas. Stringent inspection-readiness policies are common with pharmaceutical and biotech manufacturers. From a...
Continuous Process Verification per FDA Process Validation Guidance
It was just about a year ago that the U.S. Food and Drug Administration published their Guidance for Industry - Process Validation: General Principles and Practices. I caught up with Emerson's Heather Schwalje, a senior consultant on the Life Sciences industry team....
Organizational Change and Process Analytical Technology and Quality by Design
On Pharmaceutical Manufacturing magazine's web site, PharmaManufacturing.com is an 8-minute video presentation given by Emerson's Melissa Herkt. She gave the presentation, Excellence by Design: Melissa Herkt on The Case for PAT and QbD at the May 11-12 2010...
Perspectives on FDA Globalization Act
In an earlier post I mentioned how Emerson's Shenling Yang had taken an assignment in Shanghai, China. She's bringing her expertise in operations management and the Syncade software gained while executing projects as a member of the Life Sciences industry team....
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.