To inoculate people worldwide as soon as vaccines are proven requires the pharmaceutical industry to ramp up quickly to unprecedented volumes of supply. And technology transfer—moving the knowledge about and the ability to produce a vaccine from development to manufacturing—is a primary determinant of how fast it happens.
In a presentation at the ACHEMA Pulse virtual conference this year, Emerson’s Alan Johnston shared how fast rollout of COVID vaccine manufacturing has changed the mindset of how quickly a new therapy can move from development to commercial manufacturing and ultimately to the consumer.
Flexible modular automation solutions have helped enable faster time-to-market expectations. From application of single use technologies measuring critical quality parameters in-bag to updating a manufacturing line for a new therapy, today’s advanced manufacturing automation supports the flexibility needed for more rapid product deployment.
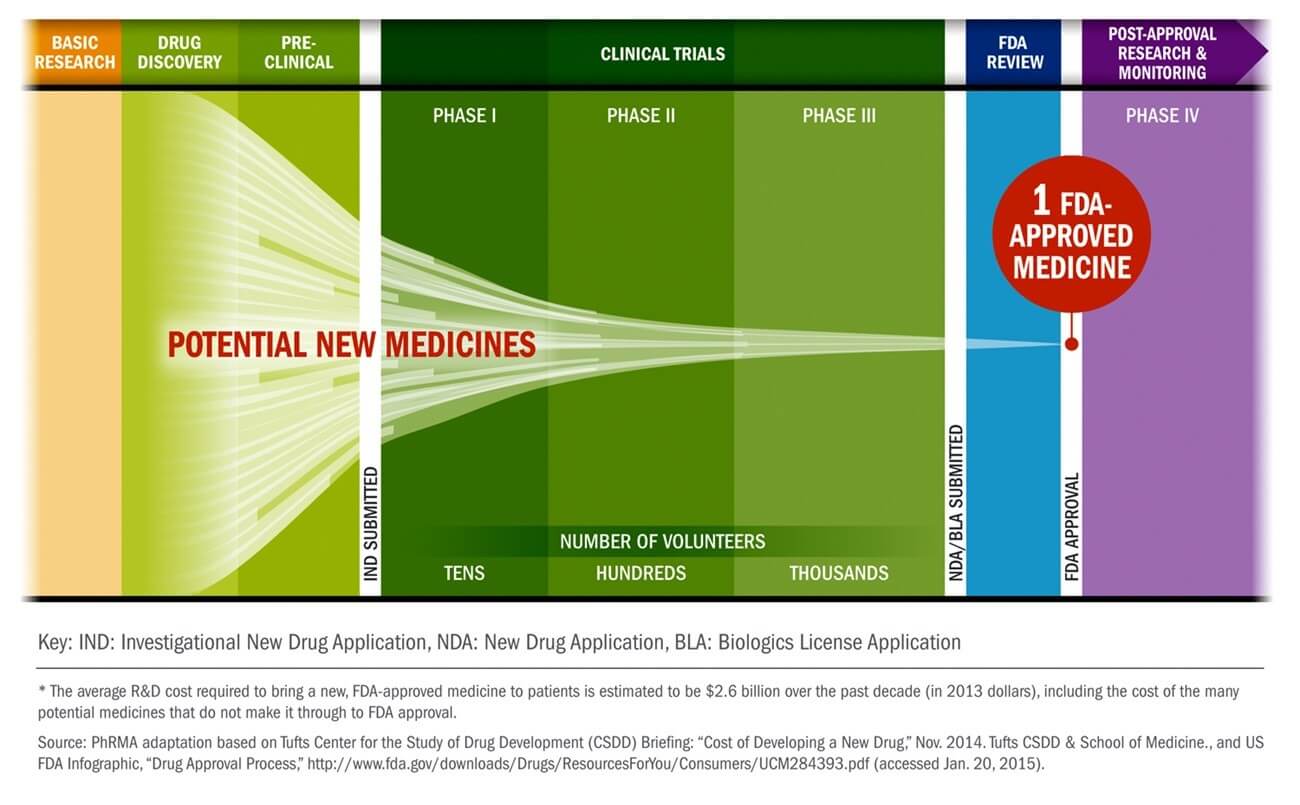
Alan opened sharing the traditional product development lifecycle through the drug development stages.
He explained that as successful compounds emerge from development, ample manufacturing capacity and a rapid scale-up of production are essential. Workstreams that are typically sequential in non-pandemic situations are being pursued in parallel. Companies are building manufacturing capacity, transferring technology, and even starting large-scale production of candidate vaccines before they have conclusive clinical data, because the economic cost of delays is so high. And because some of these vaccines will fail, manufacturers will need to ramp up even more capacity than might eventually be used, making this tremendous challenge even more difficult.
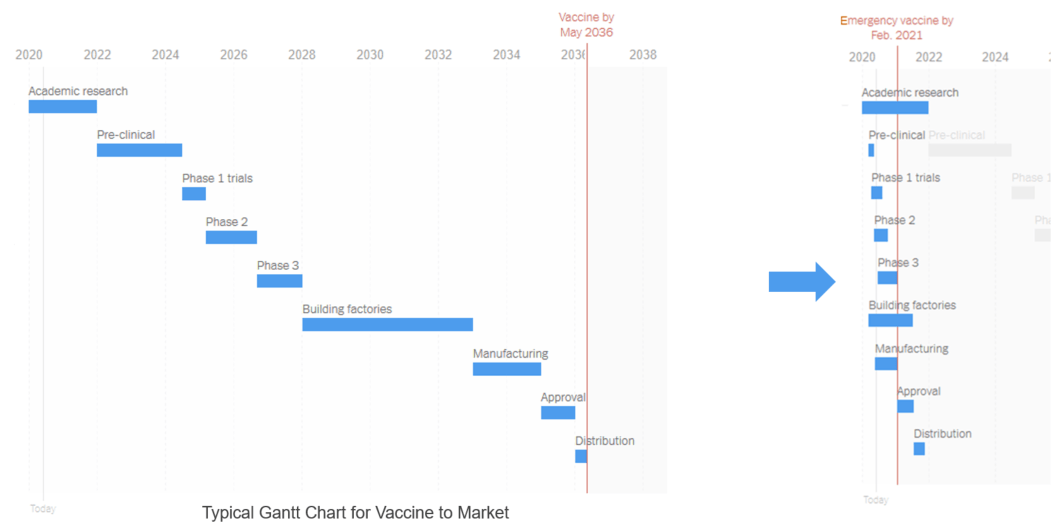
The largest acceleration opportunity is in managing and structuring tech transfer through the lifecycle phases as it correlates directly into accelerating time-to-market. Alan showed a comparison of a traditional time-to-market versus what was accomplished with the COVID-19 vaccines.
Undertaking a digital transformation initiative can yield business performance improvements in pipeline acceleration, manufacturing flexibility, real-time and intrinsic quality, and operational integrity. The analyst firm McKinsey has cited quantitative improvements from digital plants:
- Cost of quality 10-20% reduction
- Forecasting accuracy 85% increase
- Time-to-market 20-50% reduction
- Maintenance cost 10-40% reduction
- Productivity 3-5% increase
- Machine downtime 30-50% reduction
- Technical profession knowledge work productivity 45-55% increase
- Inventory holding costs 20-50% reduction
Silos of knowledge are often the greatest impediment to improved performance. The silos include labs, devices, drug substances, pilot plants, drug products, automation engineering, contract manufacturers, supply chain, manufacturing plants, quality and regulatory reporting. All of this massive amount of unrelated data makes organization, assessment and action difficult.
Achieving pipeline acceleration requires:
- Flexible recipe design and execution across scales
- Modular and flexible building blocks
- Centralized recipe management tools
- Product lifecycle change management
- Knowledge capture throughout the product lifecycle
- Harvest knowledge forward and back across lifecycle
Many factors go into supporting pipeline acceleration including business and organizational structure, risk management tools, standards and controls, process knowledge management tools, analytics across the lifecycle, and organized data.
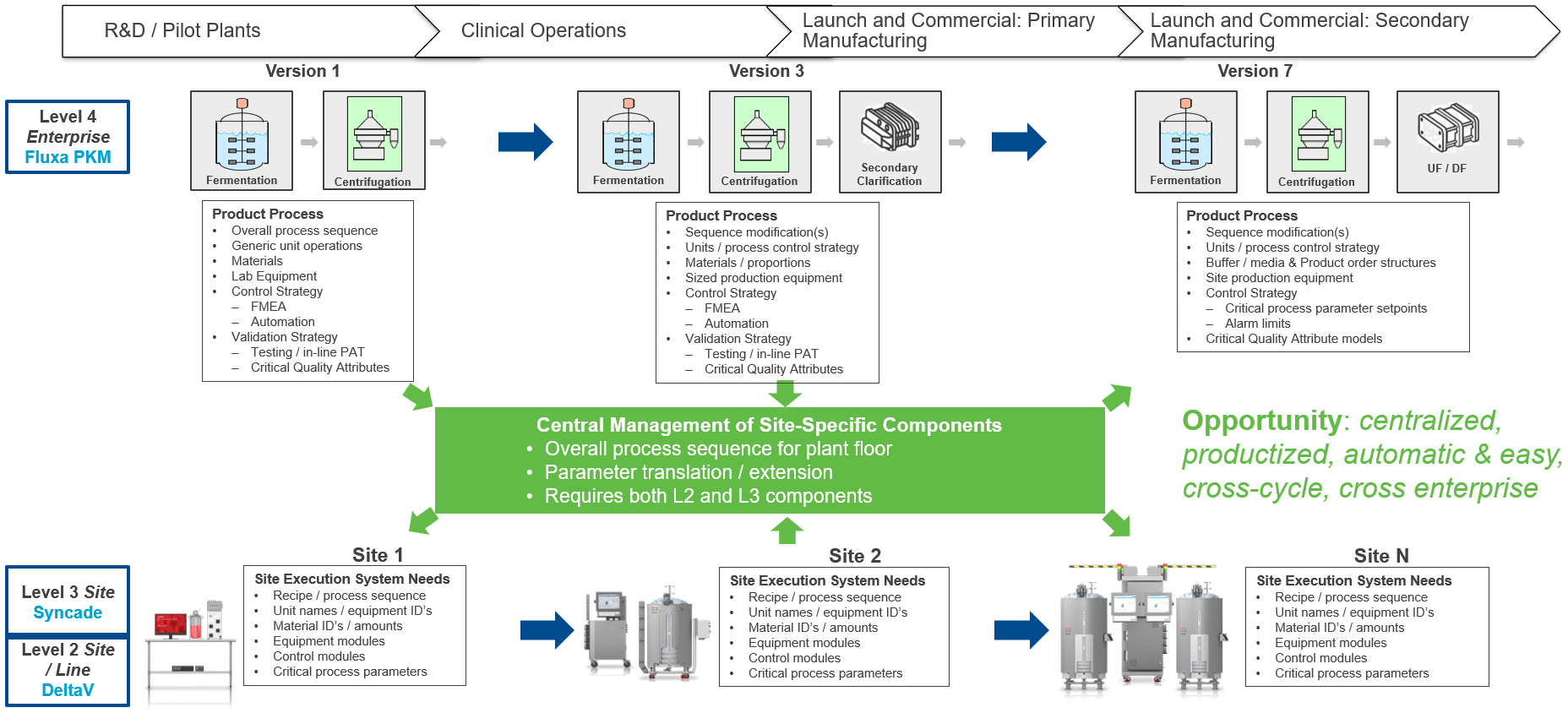
Alan showed some of the technologies and solutions that can help support faster tech transfer through the lifecycle including Fluxa PKM [Process Knowledge Management], Syncade MES (Manufacturing Execution System), and the DeltaV distributed control system (DCS).
He described other enabling technologies for pipeline acceleration. Module Type Package (MTP) reduces time and cost to integrate distributed process and reliability assets and equipment, helps accelerate time to market, simplifies fulfillment of individualized customer requirements, and improves scalability and flexibility.
Single-Use technologies (SUT) for primary and secondary processing include measurement instrumentation, valves, and control system solutions to optimize production, improve product quality, and increase efficiency.
Platforms such as Plantweb Optics securely and efficiently connect and collects global operational data from manufacturing, process control, and IT systems without disruption, contextualizes it, and transforms it into actionable information. This actionable information is presented to every decision-maker on any device, anytime, at any location.
Finally, Remote Virtual Office (RVO) cloud-based engineering can transform capital project delivery through virtualized engineering, testing, collaborative supplier project engagement, commissioning, and plant staff training.
Visit these links as well as the Life Sciences & Medical section on Emerson.com for more on the technologies and solutions to help you drive improved time-to-market performance.