With the world’s attention focused on the Coronavirus, I wanted to focus on some of the amazing therapies being developed in the Life Sciences industry. I caught up with Emerson’s Alan Johnston who shared a presentation he gave at the Simposio AFI last year.
He discussed gene therapy, cell therapy and CAR-T which combines both. These have the goal to treat disease by changing the blueprint to our body at a microscopic level. This is done by using minute pieces of ourselves to repair problems that may cause disease. This approach is designed to fix problems at their source and potentially provide a permanent cure rather than a lifetime of treatment.
Gene therapy corrects defective genes or missing genes to provide a solution for genetic disorders. It introduces healthy genes to treat or prevent diseases through replacement of the mutated gene to eliminate it or to enhance immunity to help protect the body against diseases. This approach has the potential to enable treatment of patients suffering from genetic disorders without using drugs or surgeries. Specifically, gene therapy works by injecting DNA or RNA into the patient, through a viral or non-viral vector, where it enters the patient’s cells in various tissues in order to be expressed.
Cell therapy uses living or genetically-modified cells to help treat a disease. The origin cells can be from the same individual—an autologous source, or from another individual—an allogeneic source. The cells are harvested from the donor and purified, expanded and transferred into the patient.
 CAR-T therapy combines the two. T-Cells are integral to the immune system and central in fighting disease, by latching onto antigens. White blood cells are collected from the patient and the T-Cells isolated. These cells undergo genetic reprogramming to express a new gene using a viral vector. These cells produce new receptors called Chimeric Antigen Receptors (CAR) trained to only target molecules on cancer cells. The CAR-T cells can now recognize antigens on the surface of specific cells including cancer cells. These cells are multiplied, packaged and infused back into the patient.
CAR-T therapy combines the two. T-Cells are integral to the immune system and central in fighting disease, by latching onto antigens. White blood cells are collected from the patient and the T-Cells isolated. These cells undergo genetic reprogramming to express a new gene using a viral vector. These cells produce new receptors called Chimeric Antigen Receptors (CAR) trained to only target molecules on cancer cells. The CAR-T cells can now recognize antigens on the surface of specific cells including cancer cells. These cells are multiplied, packaged and infused back into the patient.
In the pursuit of treatments for the Coronavirus, several biopharmaceutical producers are pursuing these methods along with mRNA that I mentioned in an earlier post to help accelerate the development and manufacture of a safe vaccine.
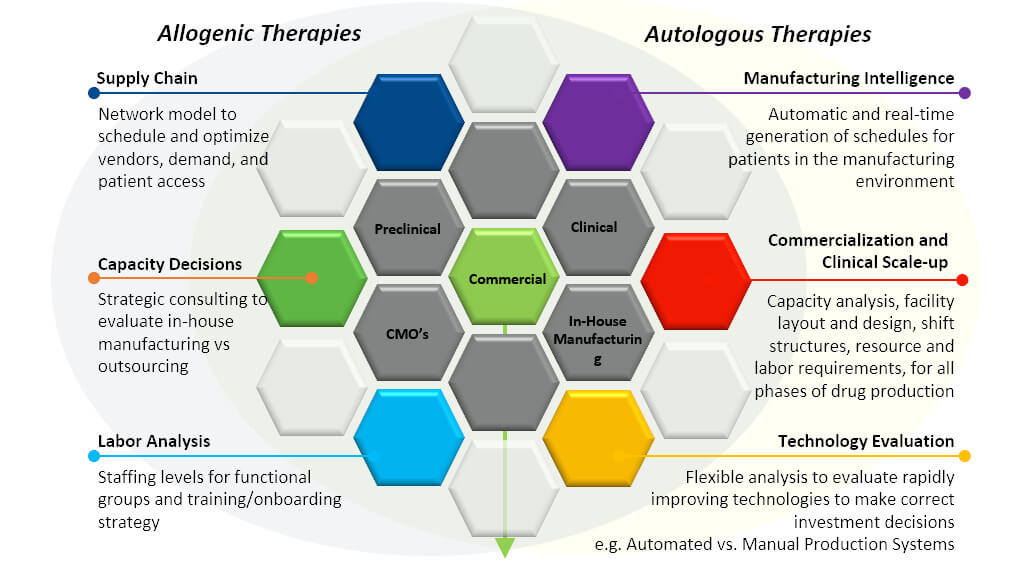
Alan noted that the manufacturing process must be affordable, accessible, high quality, of sufficient capacity, and able to be commercialized. Some tools that can help meet these manufacturing challenges include modeling, finite scheduling & optimization applications, process automation systems, and manufacturing execution systems.
For CAR-T cell therapy, the chain of identity must be assured through the process from patient to production and back to the patient. Therapy producers must manage many batches simultaneously and at various stages of the manufacturing process. Batches must have the right actions performed and recorded with the batch. And given this very manually-intensive process, entries and actions must be captured and associated with the batch records.
The combination of automation, manufacturing execution and scheduling along with single-use sensors and control elements will continue to advance to more fully automate these manual processes to deliver on the need for safe, effective and affordable CAR-T therapies.
Visit the Life Sciences & Medical section of Emerson.com for more on some of the technologies and solutions to help pharmaceutical and biopharmaceutical producers get much needed therapies to market sooner. You can also connect and interact with other experts in the Life Sciences industry in the Life Sciences group in the Emerson Exchange 365 community.





