From a recent Life Sciences symposium, we looked at issues in the advancement of process intelligence and analytics. Today we’ll look at another work session from the symposium, Managing Data Through the Product Life Cycle.
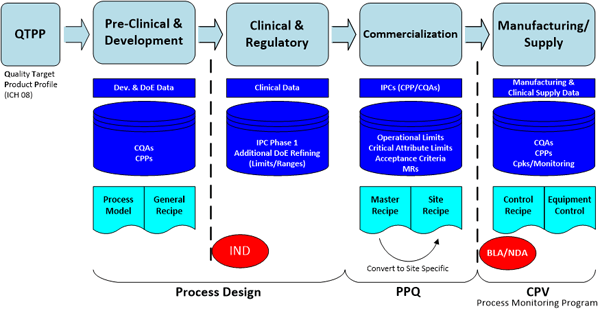
The general session was led by Amgen’s Harmik Begi and Emerson’s Michalle Adkins. The focus of the team addressing these challenges were to explore the end-to-end value chain of product lifecycle data with context as pharmaceutical and biopharmaceutical products move from research through development, clinical, and commercialization, to patients and back, including external partners.Some issues considered were the required standards and taxonomy around the data, requirements for collaboration and knowledge management, and a product focus including management of specifications, recipes, critical parameters, process narratives, etc., and linkages for technology transfer, submission and regulatory processes.
Some of the factors driving pharmaceutical and biopharmaceutical manufacturers to explore improvements in managing data through the lifecycle of projects are the need to reduce patient risks and improve health benefits, reduce process/product development time and cost, reduce product launch and technology transfer time and effort, and improve product specification management & change control with bi-directional information flow across the value chain.
From a workflow and knowledge perspective enabling internal and external product & process collaboration, making informed decisions early and quickly, and enhancing product lifecycle knowledge management: raw material, suppliers, manufacturing and distribution, patient compliance, providers, cost, patient experience and feedback are important.
And from a compliance and quality perspective enforcing compliance to regulatory filings from research to clinical to filing to commercial to patient including raw material and lot specific supply chain information and ensuring end-point quality (CQAs) through scientific analytics, Quality by Design, Continuous Process Verification, end-to-end tracking and tracing of the data to control Critical Process Parameters, and Raw material variation management is required.
Additional team members helped to lead each break out session where each group discussed these challenges with the intent to develop guidelines, standards and best practices, share case studies, and identify gaps to drive technology roadmaps among suppliers across the ISA-95 enterprise-control hierarchy.
A key part of the discussions centered around vocabulary management since it is a foundational capability that enables and informs higher-order capabilities such as metadata, tagging, search, content classification, text-mining, big data analytics, and machine learning.
Arriving at a common vocabulary also would improve the user experience for the applications involved in managing the data. Some examples where this would help is in consistent software “dropdown lists”, auto-tagging of knowledge assets such as documents, faceted searches, and analytic outputs.
Given the large scope of the effort, breakout groups were established to review frameworks, standards & ontology (formal naming and definition of the types, properties, and interrelationships of the entities that fundamentally exist for a particular domain of discourse), and driving culture change.
Michalle noted that bringing the diverse perspectives together is helping to better distill the requirements and priorities in advancing the technologies and work practices to better and more efficiently manage the data through the product lifecycle. This team will be continuing to work on these challenges as we prepare for the next Symposium event.
You can connect and interact with other pharmaceutical and biotech experts in the Life Sciences group in the Emerson Exchange 365 community.