Last week, I highlighted Emerson’s Heather Schwalje thoughts on Continuous Process Verification per U.S. Food & Drug Administration (FDA) guidelines. In a comment, Pharmaceutical Manufacturing magazine senior editor, Paul Thomas, highlighted a great article, A Practical Roadmap to Pharmaceutical Process Validation for Pharmaceutical and Biotech manufacturers.
I saw another great analysis piece by Heather on the ASTM E2500 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment.
Traditionally, the Quality organizations were not part of the new automation equipment selection process until turnover from supplier and/or engineering testing. This approach led to redundancy in documentation and testing, and caused delays in project schedules from changes made in the process to meet quality expectations.
In 2002, the FDA announced its initiative for the 21st century, which introduced the concept of Risk Management and Quality by Design. The International Conference on Harmonization (ICH) developed and released several quality guidance documents expanding upon these concepts (ICH Q8, Pharmaceutical Development and ICHQ9, Quality Risk Management). In alignment with these concepts, ASTM 2500 defines an approach where Quality oversight is introduced during the initial design phase and is continuous throughout the lifecycle.
The ASTM process introduces the concept of “Verification” instead of Qualification for manufacturing systems and leverages vendor documentation to minimize end user testing for a portion of testing and focus the effort for testing in alignment with highest risk items. Like the risk-based approach that is an integral part of the IEC 61511 safety lifecycle, the ASTM process describes a risk- and science-based approach to testing and verification based on critical aspects of manufacturing and Quality by Design.
Risk is assessed based on impact to product quality and patient safety. The level of testing is commensurate with the risk and is defined based on scientific knowledge base for the associated products with defined acceptance criteria. Traceability of design elements back to specific requirements will assist in determination of specific test cases where multiple design features may be grouped for testing if they achieve a common requirement.
Heather noted that early in the project during the requirements definition and design phases, there is a need to have input from the Quality organization and technical product subject matter experts. This process may require more stringent documentation and approvals earlier in project phases. Also, the risk assessment procedures and associated time interval to execute this risk assessment process should be factored into the overall project timeline and planning of resources. While this may extend early stages of the project, it should expedite the timeline in later phases and allow for quicker turnaround for release of systems.
Since the ASTM process includes the use of vendor documentation and Good Engineering Practices (GEP) then the vendor requires an acceptable quality system, technical capability, and application of GEP. A process manufacturer’s intent to implement the ASTM model requires an audit of the vendor’s quality system. If the vendor’s documentation is to be used to reduce the process manufacturer’s testing process, then all test plans should include pre-defined acceptance criteria and be executed using Good Documentation Practices. Items identified as high severity or high risk to product quality or patient safety may still require end-user testing to confirm vendor testing. This would not remove requirements for GMP [Good Manufacturing Practice] documentation from the vendor.
Heather described considerations for the ASTM E2500 program element concerning change management over the project and product lifecycle. In order to achieve control and implement an ASTM approach, change management is required beginning with the design phase. The ASTM model includes Quality oversight of changes beginning in the design phase for changes associated with requirements identified as Quality-impacting during the risk assessment process.
Changes impacting other areas are required to be tracked but may not be elevated to the Quality Unit. Control of changes ensures the verification process remains integral. Project work should ensure an appropriate change management program is defined during the design phase and an approach for obtaining appropriate approvals for changes is identified and agreed upon up front.
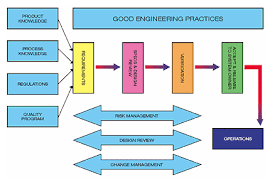 Heather summed up her thoughts referring to the ASTM E2500 process flow. The definition of requirements, as an initial step, includes input from all stakeholders including Quality and should be based on detailed process descriptions with a defined process control strategy and Critical Quality Attributes for associated products.
Heather summed up her thoughts referring to the ASTM E2500 process flow. The definition of requirements, as an initial step, includes input from all stakeholders including Quality and should be based on detailed process descriptions with a defined process control strategy and Critical Quality Attributes for associated products.
Risk analysis should begin upon identification of initial requirements to ensure appropriate specification and design documents are produced in alignment with the verification approach. Application of the risk assessment should also be consistent over the project to achieve the benefits of the ASTM E2500 approach.
Factory and site acceptance testing, upon completion of design and build, will be a direct input to verification documentation. Upon completion of all end-user testing, Quality performs an assessment for acceptance and release of the system for use in GMP operations. In a start-up project, the Quality unit may define an approach where systems may be utilized “at risk” prior to final release for start-up activities.
