We have witnessed an incredible change the last few years in how life sciences manufacturers deliver treatments. Today’s focus is on fast results, and that means products patients rely on cannot sit on shelves for weeks or months waiting on quality validation.
In a recent article in Pharmaceutical Processing World, Emerson’s Bob Lenich and Bruce Greenwald explored a strategy plants are using to digitally transform life sciences manufacturing to accomplish automated real-time quality release through closed-loop process verification and control. The underlying system—process analytical technology (PAT)—has been around for quite a while. However, a new strategy, embedded PAT, is making it far more intuitive to implement.
Quality control takes time
When treatments have completed the manufacturing process, they are still many stages out from being complete.
“Ensuring treatments are ready for patient use always necessitates running tests requiring complex multivariate data. Unfortunately, many of these tests—glucose levels, cell density, concentration of active ingredient, pH, dissolved oxygen and many more—are run manually today.”

Integrated process analytical technology can help dramatically reduce time to market.
But it isn’t just the time to manually sample a batch that delays a release. The process typically looks something like this:
- Take a sample
- Deliver the sample to the lab
- Run tests (manually)
- Wait for results
- Validate test results
- Approve the batch
If at any point there is human error in the process, it is delayed even further.
Traditional PAT is complex and fragile
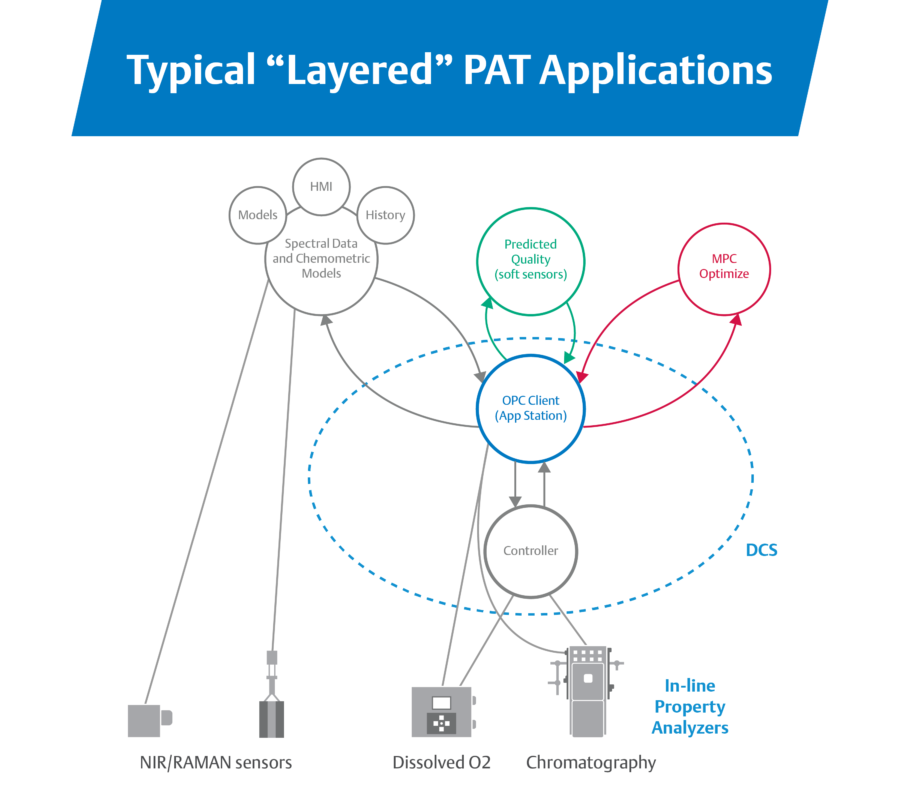
Traditional PAT unlocks closed-loop process control, but also tends to be complex to configure and maintain.
PAT helps reduce the time spent in quality steps and the risk of human error by bringing analyzer data into the control system for closed-loop process control. Instead of waiting for human intervention to verify quality after production, quality is monitored throughout the process and production teams can adapt to keep batches in line. However,
“This configuration creates a complex web for IT and OT to implement and maintain. It also creates a wide surface area for compatibility problems because changing any one element risks breaking something else. Moreover, validating all these systems is very complex and time-consuming.”
But today there is a better way.
Real-time Integrated PAT
Implementing integrated PAT in real time simplifies implementation and maintenance of closed-loop process control and dramatically shortens the quality validation process.
“To reduce complexity and design more stable closed-loop process control, organizations are collapsing the traditional architecture with integrated PAT. Using PAT embedded in the control system, operations teams bring spectral signals directly into control system blocks via OPC UA”
By performing spectral analysis in-line with production, plants can fully automate production. A function block in the control system processes spectral array signals and uses them to directly perform quality attribute calculations for real-time monitoring.
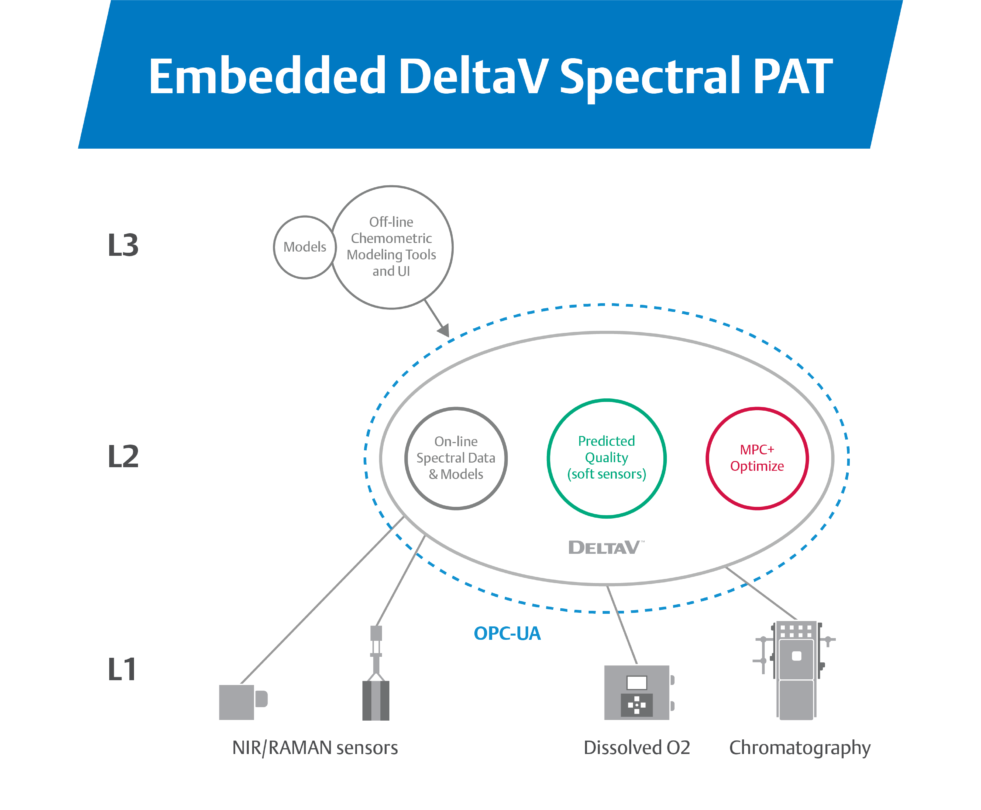
Embedded PAT simplifies the architecture of closed-loop process control, making it easy to set up and maintain.
Spectral PAT
Emerson’s DeltaV™ distributed control system reduces the complexity of closing the loop on process control. An intuitive architecture that is easy to implement and maintain enables operators to monitor multivariate data from the same interface they use to perform all other control steps. Working in a single, integrated environment makes the system more secure and easier to validate.
You can learn more about how Spectral PAT simplifies quality validation to improve speed to market by reading the article in its entirety at Pharmaceutical Processing World. And while you’re here, I’d love to hear more about the ways you are simplifying quality control in your facility.





