Professionals across the Life Sciences industry are gathering this week in New York City for Interphex. Organizers bill this conference as:
…the premier pharmaceutical, biotechnology, and device development and manufacturing event where you can “Experience Science through Commercialization”.
Emerson’s Michalle Adkins will be presenting, Update on BioPhorum’s Roadmap for In-line Monitoring and Real- Time Release at the conference. I highlight a few of the key ideas she’ll share in the presentation.
She describes the purpose of BioPhorum as a unique global collaboration to be a powerful vehicle for change. Life Sciences industry leaders and experts working in concert with the goal to deliver results by pooling knowledge, practices and ideas. Their mission:
To create an environment where the global biopharmaceutical industry can collaborate and accelerate their rate of progress, for the benefit of all.
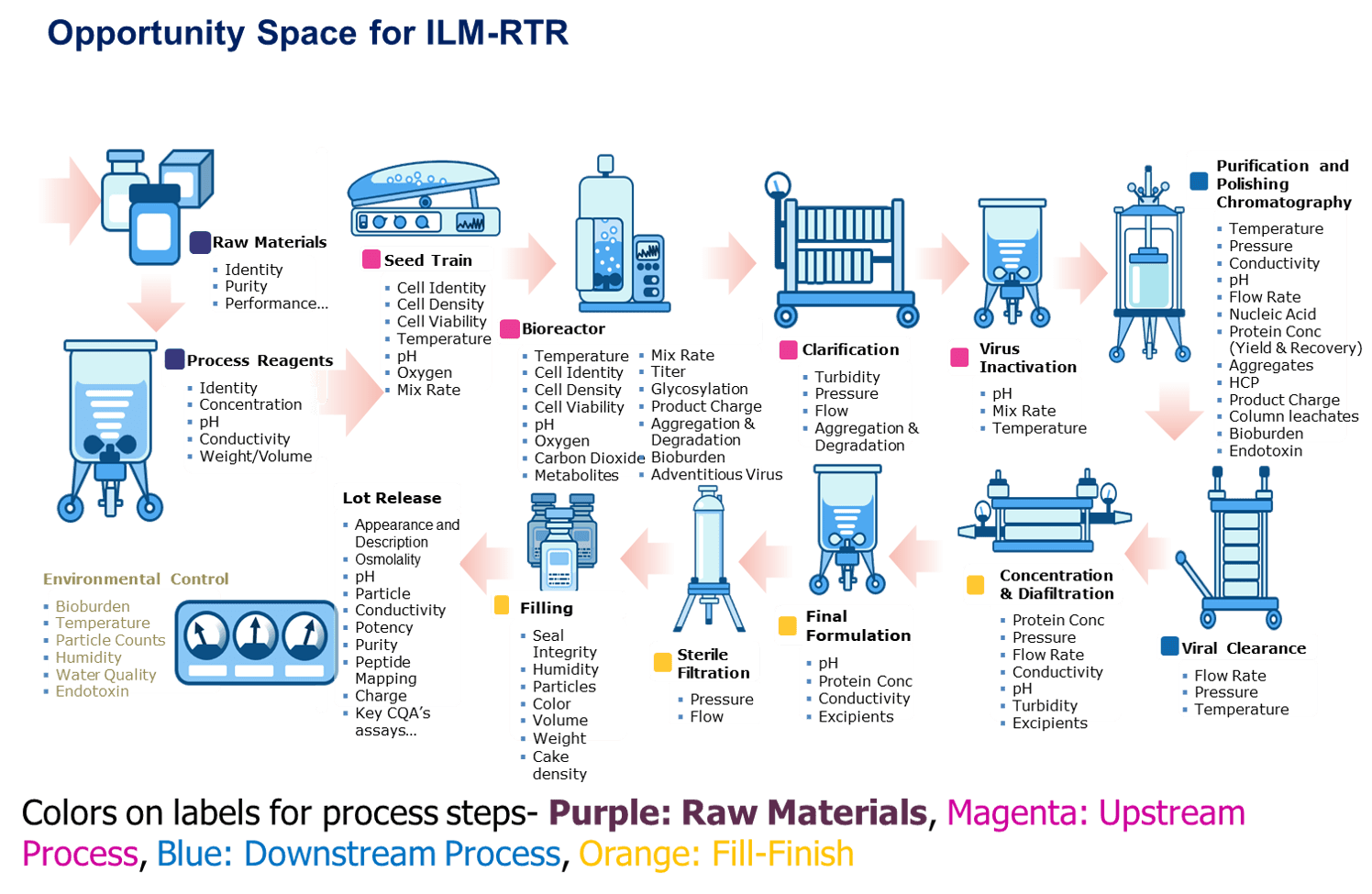
Image courtesy of BioPhorum Operations Group from the BioPhorum Technology Roadmap for the Biopharmaceutical Manufacturing Industry.
To this end, the BioPhorum Operations Group published the first edition of a technology roadmap. This roadmap is the result of two years effort by 31 member companies and driven by BioPhorum’s Technology Roadmapping Steering Committee and its six enabling technologies roadmap teams. Sign up to request the executive summary of this roadmap.
Michalle will highlight some trends in biopharma development and manufacturing including the continued move towards a leaner state to increase yield, efficiency and throughput while also reducing waste, lead-time and compliance risk.
The objectives of the roadmaps are to:
- Increase product and process understanding
- Reduce time needed to introduce process changes
- Reduce time required to manufacture and release product
- Reduce total cost of supply
- Reduce cost of upfront investment in manufacturing
- Reduce cost of development
In-line Monitoring & Real-time Release (ILM-RTR) provide many opportunities to transition analysis from off-line testing to real-time testing using technologies such as advanced chromatography, sensors, and non-invasive methods of measurements. These opportunities extend from raw materials through lot release and the processing steps in between. The goal is to produce a prioritized list of critical quality attributes (CQAs) and in-process controls (upstream to drug substance) to inform the CQAs that should be targeted for a transition to in-line, on-line or at-line monitoring.
If you’ll be at Interphex this week, make sure to catch Michalle in the Emerson booth #3453. Michalle’s presentation on Wednesday April 3 at 1:15pm on Stage 1 in Booth 1076 where she’ll provide these updates and describe the process of how this goal is being achieved.
If you can’t make the conference, make sure to visit the Life Sciences & Medical section on Emerson.com and join your peers in the Life Sciences group in the Emerson Exchange 365 community.
Update: The date of Michalle’s presentation was incorrect in the program on the Interphex website and was Tuesday. If you’re at Interphex 2019, you can connect with Michalle in the Emerson booth #3453.
