Exceptions in batch manufacturing processes are the deviations that occur outside the prescribed specifications. For pharmaceutical and biopharmaceutical manufacturers, quality and manufacturing personnel must review these exceptions. Traditionally, this quality review process occurs after the batch has completed.
A recent news release, Emerson Helps Life Sciences Companies Reduce Time to Market with Faster Quality Reviews, announced enhancements to the Syncade manufacturing execution system (MES) to streamline this review-by-exception process, turning weeks into days for quality reviews.
Emerson’s Kevin Stembridge, a Syncade product marketing director, noted that the Syncade Quality Review Manager application shortens the release process by simplifying and streamlining the process of accurately documenting all actions taken when responding to exceptions.
Sources of these exceptions can come from measurements and final control actions orchestrated by the DeltaV distributed control system, Syncade Workflow, or other third party systems that generate process-related exceptions.
By integrating these different data sources involved in the batch manufacturing process with a manufacturer’s exception review process, overall data integrity is improved and compliance with regulations—U.S. FDA 21 CFR Part 11 and EU Annex 11—is simplified.
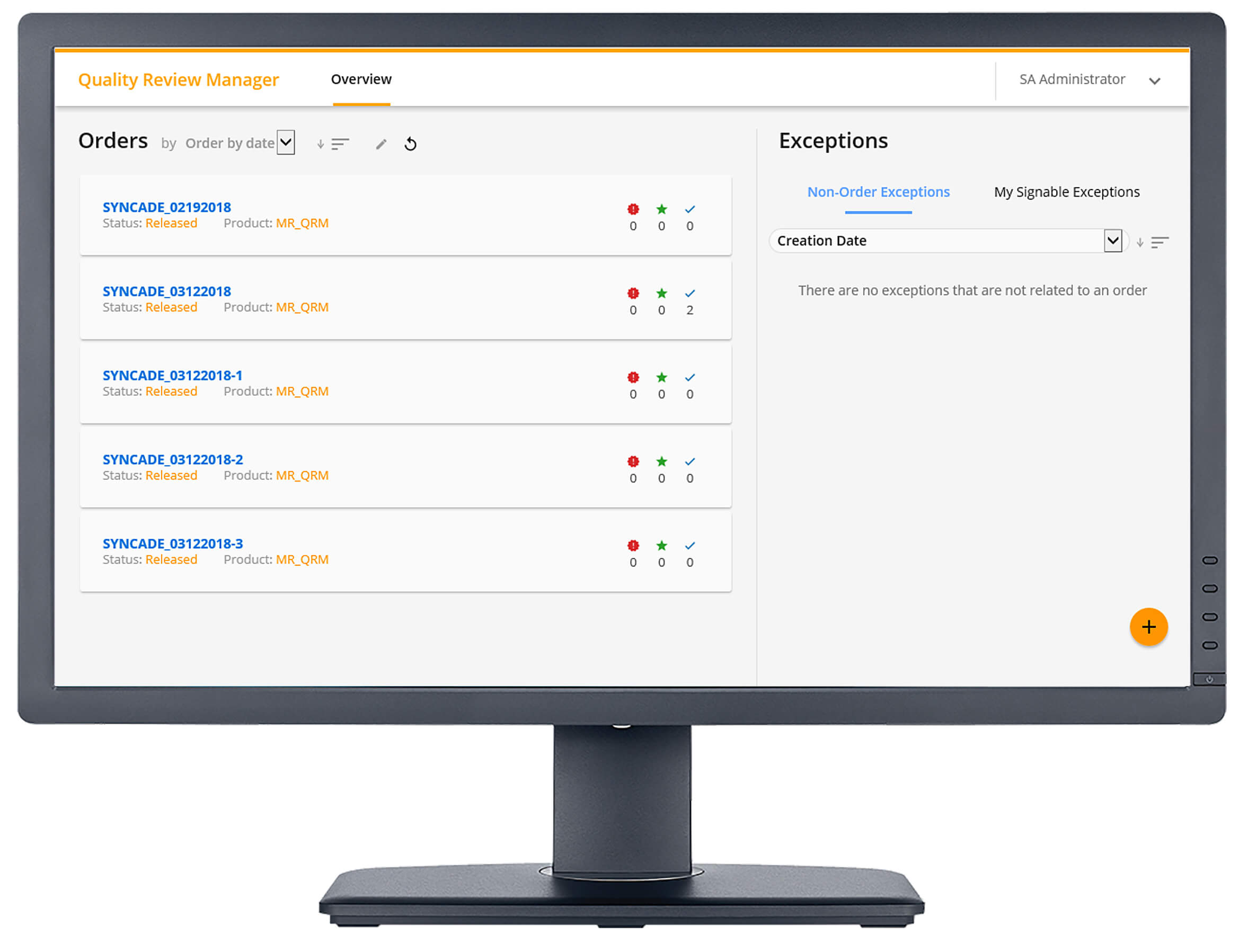 With the Quality Review Manager, the quality department can review process exceptions as they occur and while the batch is still in process, giving a more accurate and timely response. Exception dashboards help prioritize reviews while focusing on the most critical exceptions affecting the process. After all exceptions are closed, Quality Review Manager supports an automated release method by immediately releasing finished batches once all exceptions are resolved, reducing the lag between production, product release and patient delivery.
With the Quality Review Manager, the quality department can review process exceptions as they occur and while the batch is still in process, giving a more accurate and timely response. Exception dashboards help prioritize reviews while focusing on the most critical exceptions affecting the process. After all exceptions are closed, Quality Review Manager supports an automated release method by immediately releasing finished batches once all exceptions are resolved, reducing the lag between production, product release and patient delivery.
Kevin described some of the captured exceptions such as issues detected with critical process parameters, non-standard activities (such as forced steps), comments are added to work instructions, DCS alarms and events, and manually-logged exceptions.
The Quality Review Manager user interface is based on HTML5 technology which delivers scalability and compatibility with current web browsers and most modern mobile tablet environments.
For more on Quality Review Manager and the other Syncade MES applications, visit the Manufacturing Execution Systems section on Emerson.com. You can also connect and interact with other manufacturing execution system and pharmaceutical & biotech industry experts in the Operations Management and Life Sciences groups in the Emerson Exchange 365 community.





