While integration technologies have helped bridge “islands of automation” over the years, some process manufacturers still have parts of their automated processes disconnected from the rest. Emerson’s Gary Mitchell, a senior industry consultant on the Life Sciences industry team, shares some recent experiences on bridging these islands.
I’m spending a lot of my time recently with both existing and potential customers wishing to improve operations at their legacy facilities by reducing the number of “Islands of Automation”. We use the phrase islands of automation to describe controls/systems that are not connected/integrated.
Islands of automation was a popular term used largely during the 1980s to describe how rapidly developing automation systems were at first unable to communicate easily with each other. Industrial communication protocols, network technologies, and system integration helped to improve this situation. Just a few of the many examples of helping technologies are Modbus, Fieldbus, Ethernet, etc. The term is now more appropriate to information technology, where Enterprise application integration looks to solve the problem of islands of automation in the IT field.
Whilst most pharmaceutical and biotech manufacturers have automated their primary manufacturing facilities, the same cannot be said for the majority of secondary manufacturing sites.
Secondary manufacturing activities for small (chemically manufactured products, the majority of drugs on the market) molecules include blending, granulation & drying, tabletting & capsulation, coating, etc.
Whereas secondary manufacturing activities for large (also known as biologics or biopharmaceuticals) molecules include formulation, fill/finish, inspection, label & packaging, etc.
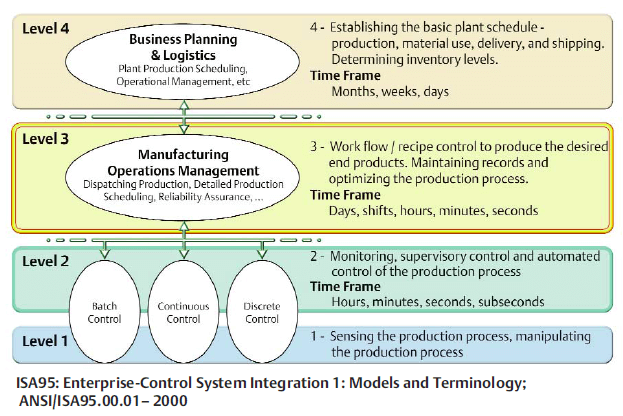
One prospective client has 10 fill/finish facilities around the globe. This manufacturer’s vision is for a future global manufacturing execution system (MES) that could talk up to ISA-95 level 4 systems such as enterprise resource planning (ERP) and data historian, as well as down to all the level 2 controls and equipment. In order to achieve these goals, a common batch automation layer will need to be added at each site due to the different configurations of OEM equipment and control systems at each site.Our team proposed their DeltaV control system as the batch automation layer, which has standard interfaces for “up” to MES/ERP/LIMS/Data Historian systems (via Campaign Manager/Recipe Exchange Web Services) and “down” to OEM skids/legacy control systems (via Serial Interface cards or Virtual I/O modules). Should this project go forward, it is anticipated that a FEED (Front End Engineering Design) would happen at each site to evaluate which controls (e.g. at the OEM skid) remain and therefore interfaces to the DeltaV system, versus which controls would move completely into the new automation layer control system.
Another project with which I was recently involved was a FEED study for a European vaccines manufacturer whose islands of automation were leading to production and regulatory issues. The accepted solution involved replacing the existing legacy control systems with Emerson’s DeltaV Batch system, as well as local bioreactor controls moving into the new DeltaV system. The remaining OEM packages/skids will be interfaced to DeltaV electronically to remove these islands of automation.
Other areas of improvement from removing “Islands of Automation” include:
- Control System Architecture—hardware obsolescence, software support, compliance issues, electronic signatures, etc.
- Vendor Packages Integration—multiple logins/security, alarm management, vendor support, etc.
- Recipe Structure—multiple recipes/batch records, frequent operator interaction, poor data capture, etc.
You can connect and interact with Gary and his fellow Life Science consultants in the Life Sciences track of the Emerson Exchange 365 community.