For those in the biotech and pharmaceutical manufacturing industries, Emerson’s Chris Amstutz has been sharing a great series of forum posts in the Emerson Exchange 365 community, Life Sciences track. Here are a few examples of some specific SAP to manufacturing execution system connectivity posts:
- SAP Transactions with Syncade in Life Sciences – Overview
- SAP Transactions with Syncade in Life Sciences – Material Master Downloads and Updates
- SAP Transactions with Syncade in Life Sciences – Production Order Download
- SAP Transactions with Syncade in Life Sciences – Transfer Order Download
- SAP Transactions with Syncade in Life Sciences – Quality Status Verification
- SAP Transactions with Syncade in Life Sciences – Goods Issue
- SAP Transactions with Syncade in Life Sciences – Miscellaneous Goods Issue
- SAP Transactions with Syncade in Life Sciences – Goods Receipt
Chris also shared with me the background behind the Good Automated Manufacturing Practice (GAMP) Category 5 software development lifecycle for validated environments. Category 5 (CAT 5) software is defined as non-COTS [commercial off-the-shelf] software (custom) that is developed specifically for the project. Since the software is not standard product, it is required to be developed according to Software Development Lifecycle (SDLC).
Chris notes that GAMP 5 provides an SDLC framework that ensures software quality traceable through requirements, development, testing, deployment, and revision. An example of CAT 5 software would be an interface between an enterprise resource planning (ERP) software package such as SAP and an operations management software package such as Syncade. This interface would be to coordinate communications between SAP and Syncade.
In developing CAT 5 software, a cross-functional team needs to be established. The team would include an overall project manager responsible for component requirements and functional design specification (FDS) approval and a quality project manager that owns and maintains of the CAT 5 SDLC procedure/adherence procedure/good testing and documentation practices. Other key roles and responsibilities include a technical lead who develops the FDS, a software developer who performs coding and maintenance, software unit, system and module & installation test plan development and testing, configuration management, and release note development.
Chris notes that the following SDLC activities and deliverables should be provided for each software component with responsibilities assigned for each activity and deliverable: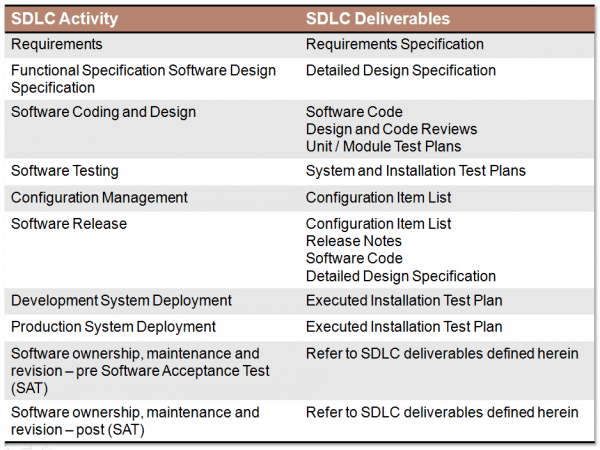
Consider joining the Emerson Exchange 365 community and the Life Sciences track to join in the conversations with your peers.




