This week, the International Foundation Process Analytical Chemistry (IFPAC) is holding their 26th international forum and exhibition in Baltimore, Maryland USA. At this event, Emerson’s Chris Amstutz, Life Sciences industry consulting team director, gave a presentation, Incorporating PAT Methods into Production Recipes for Real Time Release.
The U.S. Food and Drug Administration defines Process Analytical Technology (PAT):
…a system for designing, analyzing, and controlling manufacturing through timely measurements (i.e., during processing) of critical quality and performance attributes of raw and in-process materials and processes with the goal of ensuring final product quality.
In his presentation, Chris notes that PAT methods are developed during the research and development or pilot plant phase. Typically, there is little or no consideration as to how the PAT methods will scale up or be deployed in production. As a result, they are not. A Pharmaceutical or Biotech manufacturer’s Manufacturing and Quality Assurance (QA) teams are reluctant to eliminate offline measurements because of their historic use in the manufacturing process. And this reliance on the tried and true extends to the operators who often do not have the confidence in the online measurement methods.
Chris listed some considerations for the use of PAT methods in production. It’s important to clarify what will be the system of record. Starting with the end in mind, determining what information QA needs in the final batch record is critical. Next, what level of interaction is needed between a shop floor recipe and PAT method? From an operator perspective, what information is wanted or needed? The answers help determine if open loop or closed loop control will be required. From a change management perspective, how are versions of the PAT methods that are in recipes managed? Finally, given the deployment of online PAT methods, is offline sample collection still required?
Chris highlighted a typical batch process with manual work instructions, automation, and offline testing. In his example, he cited a moisture test where after an automated 15-minute drying phase, a manual sample is taken and tested for moisture content. If not dry enough, the drying step runs an additional minute and is retested. If the moisture level is within specification, the batch proceeds to the material transfer step. The constraint in this process is the sampling and manual testing.
With an embedded PAT method, the dry material “start dryer” step initiates the PAT method, which performs continuous moisture testing. Once the specification level is reached the PAT method ends and “stop dryer” step begins.
Chris counseled to treat the PAT method like a phase and keep it simple. It should be sequenced in a common recipe-authoring tool. The PAT method performs an action without operator intervention. The design of this method should provide a similar user experience to what operators see every day to keep it familiar. The results of the PAT method should be in the batch end report, just like all the other analyses performed. Like any process upset condition, an alarm should be raised if a problem occurs during the PAT method.
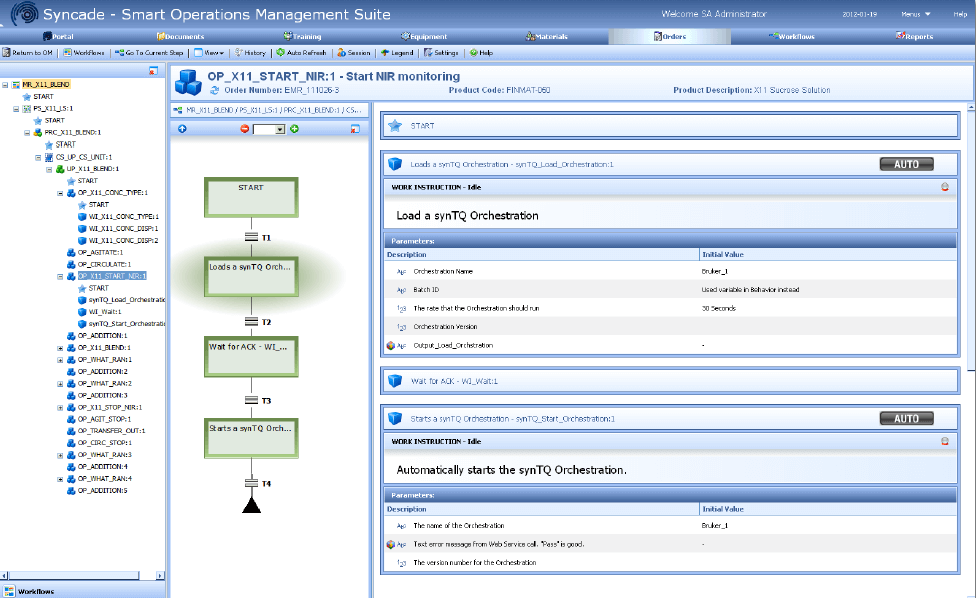 Chris described the process and components of how this recipe execution occurs. The recipe is initiated in Syncade operations management software. The PAT method, base on synTQ Orchestrations, are launched based upon the built in recipe triggers. Parameters are passed from the Syncade software to the PAT methods base upon the method’s requirements. During the recipe’s execution, communication between the Syncade software, DeltaV system, and PAT method are providing operators the picture of manual and automated steps as the recipe advances. As the PAT method completes, the details are incorporated into the final batch record.
Chris described the process and components of how this recipe execution occurs. The recipe is initiated in Syncade operations management software. The PAT method, base on synTQ Orchestrations, are launched based upon the built in recipe triggers. Parameters are passed from the Syncade software to the PAT methods base upon the method’s requirements. During the recipe’s execution, communication between the Syncade software, DeltaV system, and PAT method are providing operators the picture of manual and automated steps as the recipe advances. As the PAT method completes, the details are incorporated into the final batch record.
By incorporating these PAT methods into production recipes, the delays associated with manual testing can be eliminated and a more complete batch record assembled to improve the quality and shrink the time until the product is ready for sale.
