You may have been seeing the phrase “Digital Twin” more and more in the automation and industry press. Wikipedia defines the Digital twin concept as:
…a digital replica of physical assets, processes and systems that can be used for various purposes.[1] The digital representation provides both the elements and the dynamics of how an Internet of Things device operates and lives throughout its life cycle.[2]
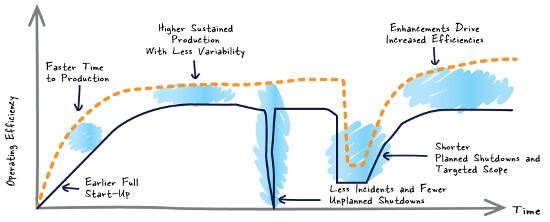
In a LinkedIn post, Digital Twin Streamlines Regulatory Compliance in Life Sciences, Emerson’s Ronnie Bains describes how a dynamic simulation provides a software-based digital twin of a production facility.
He notes that these dynamic simulations have not been traditionally been deployed in pharmaceutical and biotech manufacturing operations [hyperlink added]:
…however using Emerson’s Mimic simulation technology, a new standard of simulation technology, perfectly suited to the Life Sciences industry is feasible.
Projects and ongoing operations can be streamlined by developing the digital twin during the initial design phase and using it in:
…a wide range of activities during the project design, commission and operate phases of a Life Sciences project.
Given the challenges pharmaceutical and biotech manufacturers face with regulatory compliance:
…the digital twin can be used to demonstrate compliance with GMP [Good Manufacturing Practice] and FDA [U.S. Food and Drug Administration] requirements to ensure products are consistently produced and controlled according to specific standards.
Ronnie will be conducting two webinars—the first on February 6 and repeated on February 13, both at 11:00 CET. He’ll share how the digital twin gets product to market quicker and minimizes risk for manufacturers in the Life Sciences industry. Join him to learn more about how to improve project and ongoing operational performance.
You can also connect and interact with other pharmaceutical and simulation experts in the Life Sciences, DeltaV and Ovation groups in the Emerson Exchange 365 community.